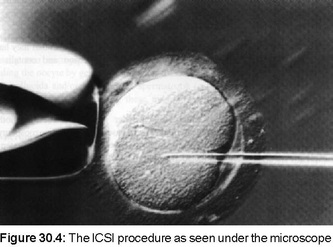
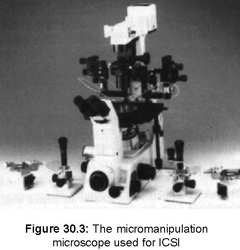
Stimulated Cycles
In cycles stimulated with HMG or FSH, serial ultrasound scanning of ovarian follicles with or without serial plasma estradiol determinations is carried out. HCG (5000 to 10000 IU) is administered when the oocytes reach a mean follicular diameter of 17–18 mm and the plasma estradiol has reached 1500 to 2500 pmol/L. Oocyte recovery is carried out 32 to 36 hours afterwards (Goswamy et al, 1984). A combination of both parameters is sometimes used (i.e. 1800 pmol/L per 18 mm follicle as described by Trounson and Conti 1982).
In cycles stimulated with HMG or FSH, serial ultrasound scanning of ovarian follicles with or without serial plasma estradiol determinations is carried out. HCG (5000 to 10000 IU) is administered when the oocytes reach a mean follicular diameter of 17–18 mm and the plasma estradiol has reached 1500 to 2500 pmol/L. Oocyte recovery is carried out 32 to 36 hours afterwards (Goswamy et al, 1984). A combination of both parameters is sometimes used (i.e. 1800 pmol/L per 18 mm follicle as described by Trounson and Conti 1982).

Transvaginal Ultrasound-directed Approach
This is now the technique of choice used by most IVF centers. It is much simpler, easier and less painful than the previous three techniques. The whole procedure can be carried out through the vagina (Feichtinger and Kemeter, 1986). A full bladder is not necessary and a special transducer is introduced through the vagina. A radial picture is obtained and the needle is introduced through one of the fornices to reach the visualized follicles. The follicular fluid is aspirated using a pump connected to the Delee trap or alternatively using a plastic syringe.
This is now the technique of choice used by most IVF centers. It is much simpler, easier and less painful than the previous three techniques. The whole procedure can be carried out through the vagina (Feichtinger and Kemeter, 1986). A full bladder is not necessary and a special transducer is introduced through the vagina. A radial picture is obtained and the needle is introduced through one of the fornices to reach the visualized follicles. The follicular fluid is aspirated using a pump connected to the Delee trap or alternatively using a plastic syringe.
Sperm Preparation
After oocyte retrieval, the husband is asked to collect his ejaculate into a sterile container. The semen is allowed to liquefy at room temperature and a seminal fluid analysis is performed. In our program this is carried out using the computerized system of Fertipro (Autosperm) which calculates the sperm count, the motility grades (a, b, and c) as well as the velocity, linear velocity and linearity index. A film of seminal fluid is also spread on a glass slide and stained arid strict morphology is calculated under oil immersion microscopy (x1000). If the normal forms calculated by strict morphology are higher than 14 percent, IVF is carried out. If these are less than 5 percent, ICSI is carried out. If the normal forms are between 5 and 14 percent, IVF is carried out on half the oocytes and ICSI on the other half. The semen is then prepared by the swim-up method or the Percoll gradient method and the concentration recalculated. The sperm concentration is then adjusted to 0.5 to 1 million spermatozoa/mL.
After oocyte retrieval, the husband is asked to collect his ejaculate into a sterile container. The semen is allowed to liquefy at room temperature and a seminal fluid analysis is performed. In our program this is carried out using the computerized system of Fertipro (Autosperm) which calculates the sperm count, the motility grades (a, b, and c) as well as the velocity, linear velocity and linearity index. A film of seminal fluid is also spread on a glass slide and stained arid strict morphology is calculated under oil immersion microscopy (x1000). If the normal forms calculated by strict morphology are higher than 14 percent, IVF is carried out. If these are less than 5 percent, ICSI is carried out. If the normal forms are between 5 and 14 percent, IVF is carried out on half the oocytes and ICSI on the other half. The semen is then prepared by the swim-up method or the Percoll gradient method and the concentration recalculated. The sperm concentration is then adjusted to 0.5 to 1 million spermatozoa/mL.
Luteal Phase Support
In patients stimulated with HMG or FSH only, support of the luteal phase has been suggested and practiced by many workers on the basis of unconfirmed studies suggesting disruption of the luteal phase following the dislodgement of the granulosa cells by repeated flushing of the follicles. However, a meta-analysis of randomized trials did not support this concept (Daya, 1988), but most workers still prefer to add progesterone supplementation in the luteal phase. In patients receiving GnRH analogues, luteal support is thought to be necessary as the analogues diminish the amount of progesterone secreted from the corpus luteum. Progesterone supplementation can be effected by the daily administration of intramuscular progesterone (50 to 100 mg/day) or by the oral or vaginal administration of micronized progesterone (400 to 600 mg/day). In our program, 600 mg of micronized progesterone administered orally every day until the pregnancy test is negative or until 14 weeks of pregnancy. This is increased 80 mg in patients receiving GnRH analogues. A quantitative test is always requested 14 days after embryo transfer and this is repeated after 4 days to determine the occurrence and progress of any pregnancy. If the test is positive, an ultrasound scan is performed 4 weeks after embryo replacement in order to determine the number of gestation sacs and the viability of the pregnancy.
In patients stimulated with HMG or FSH only, support of the luteal phase has been suggested and practiced by many workers on the basis of unconfirmed studies suggesting disruption of the luteal phase following the dislodgement of the granulosa cells by repeated flushing of the follicles. However, a meta-analysis of randomized trials did not support this concept (Daya, 1988), but most workers still prefer to add progesterone supplementation in the luteal phase. In patients receiving GnRH analogues, luteal support is thought to be necessary as the analogues diminish the amount of progesterone secreted from the corpus luteum. Progesterone supplementation can be effected by the daily administration of intramuscular progesterone (50 to 100 mg/day) or by the oral or vaginal administration of micronized progesterone (400 to 600 mg/day). In our program, 600 mg of micronized progesterone administered orally every day until the pregnancy test is negative or until 14 weeks of pregnancy. This is increased 80 mg in patients receiving GnRH analogues. A quantitative test is always requested 14 days after embryo transfer and this is repeated after 4 days to determine the occurrence and progress of any pregnancy. If the test is positive, an ultrasound scan is performed 4 weeks after embryo replacement in order to determine the number of gestation sacs and the viability of the pregnancy.
Frozen Embryo Transfer
If you have frozen embryos you may have decided to try to conceive using these (Rizk 2008, 2009). This procedure is called a Frozen Embryo Transfer. As the embryos have already been created and stored, the preparation is much easier for this procedure compared to an IVF cycle.
The beginning of the process is very similar to an IVF protocol. You will be instructed to report the start of your period so that an US and lab-work can be done on or around the third cycle day. At that time birth control pills are started and the patient is given a date to begin leuprolide injections. This is begun at a date and dose recommended by the physician. Usually the birth control pills are overlapped with the leuprolide for several days and then discontinued. A period should begin during the first ten days of leuprolide therapy and an ultrasound and more lab-workisdonenearthetenthdayofthistreatment. Once this is completed, you will be started on estrogen patches, pills, or injections or a combination of these. In addition, you will continue your leuprolide. An ultrasound and estrogen level will be done during the protocol to check the thickness of the uterine lining. At a certain point in your plan, you will begin progesterone injections and stop the leuprolide. If all is well with lab and ultrasound results, a transfer date will be scheduled. The transfer itself is no different than your first embryo transfer and recovery and any other activity restrictions your doctor advises are usually the same. You will continue all medications except leuprolide until your pregnancy test is completed. If you are pregnant, your doctor will tell you how long to continue these medications and what, if any, dosage adjustments to make
If you have frozen embryos you may have decided to try to conceive using these (Rizk 2008, 2009). This procedure is called a Frozen Embryo Transfer. As the embryos have already been created and stored, the preparation is much easier for this procedure compared to an IVF cycle.
The beginning of the process is very similar to an IVF protocol. You will be instructed to report the start of your period so that an US and lab-work can be done on or around the third cycle day. At that time birth control pills are started and the patient is given a date to begin leuprolide injections. This is begun at a date and dose recommended by the physician. Usually the birth control pills are overlapped with the leuprolide for several days and then discontinued. A period should begin during the first ten days of leuprolide therapy and an ultrasound and more lab-workisdonenearthetenthdayofthistreatment. Once this is completed, you will be started on estrogen patches, pills, or injections or a combination of these. In addition, you will continue your leuprolide. An ultrasound and estrogen level will be done during the protocol to check the thickness of the uterine lining. At a certain point in your plan, you will begin progesterone injections and stop the leuprolide. If all is well with lab and ultrasound results, a transfer date will be scheduled. The transfer itself is no different than your first embryo transfer and recovery and any other activity restrictions your doctor advises are usually the same. You will continue all medications except leuprolide until your pregnancy test is completed. If you are pregnant, your doctor will tell you how long to continue these medications and what, if any, dosage adjustments to make

Embryology Lab
Ovum Handling : The aspirated follicular fluid is directly examined under the magnifying power of a stereomicroscope (×10 to ×40) for the presence of oocytes. These are usually surrounded by one layer of nursing cells (the corona radiata) and both enclosed in a mass of granulosa cells (the cumulus oophorous). The oocyte-granulosa-cumulus complex (OGCC) measures about 1 mm in diameter and can readily be identified by the naked eye. The OGCC appears as a silvery shining dot suspended in the follicular fluid but must be confirmed by examination under the stereomicroscope to ensure that an ovum is embedded within the cumulus.
The OGCC is washed twice in the culture medium and is transferred into another droplet of equilibrated medium under sterile paraffin oil in a special Petri dish.The oocyte is also examined for signs of maturity and the time of insemination is determined accordingly. The insemination is usually performed 4 to 6 hours after oocyte retrieval, but this can be delayed for up to 24 hours if the oocyte is thought to be immature. Signs of maturity of the oocyte include the presence of the first polar body and the dispersion of the cumulus. An immature oocyte is tightly surrounded by the granulosa cells so that its internal details are not clearly recognized.
Sperm Preparation : After oocyte retrieval, the husband is asked to collect his ejaculate into a sterile container. The semen is allowed to liquefy at room temperature and a seminal fluid analysis is performed. In our program this is carried out using the computerized system of Fertipro (Autosperm) which calculates the sperm count, the motility grades (a, b, and c) as well as the velocity, linear velocity and linearity index (Chapter 9). A film of seminal fluid is also spread on a glass slide and stained arid strict morphology is calculated under oil immersion microscopy (x1000). If the normal forms calculated by strict morphology are higher than 14 percent, IVF is carried out. If these are less than 5 percent, ICSI is carried out (Chapter 19). If the normal forms are between 5 and 14 percent, IVF is carried out on half the oocytes and ICSI on the other half. The semen is then prepared by the swim-up method or the Percoll gradient method as described in Chapter 17 and the concentration recalculated. The sperm concentration is then adjusted to 0.5 to 1 million spermatozoa/mL).
Fertilization : A volume of 100 microlitres of the processed semen (containing 50,000 to 100,000 spermatozoa) is added to the droplet of medium containing the oocyte. Different culture media have been successfully used (e.g. Ham F10, Earle’s balanced salt solution, Menezo B2, etc.). They all consist of a balanced salt solution supplemented by essential amino acids and other nutrients. The osmolarity of the culture medium is about 280 mOsm/L and the pH is 7.4 if kept in an atmosphere containing 5% CO2. The culture is carried out in special polystyrene plastic tubes or dishes. If the culture is carried out in a dish, the medium is sometimes covered with a thin of sterile paraffin wax to minimize evaporation and hence keep the osmolarity constant.
The culture containing the oocytes and spermatozoa is transferred to a CO2 incubator which keeps the temperature at 37°C and an atmosphere of 5% CO2 in air. Alternatively an atmosphere of 5% CO2 + 5% O2 + 90% N2 is used. This ensures that the pH of the culture is kept at 7.4. Six to eighteen hours after insemination, the oocytes are denuded from the surrounding granulosa cells using finely pulled Pasteur pipettes and are then examined for signs of fertilization. At this stage, fertilization is confirmed by the finding of 2 pronuclei in the cytoplasm of the oocyte (the 2 PN stage, i.e. the 2 pronuclei stage). Other signs of fertilization are:
1. The appearance of an extruded second polar body in the vitelline space.
2. Retraction of the cytoplasm of the oocyte away from the zona pellucida.
3. The appearance of a sperm head, midpiece and tail inside the cytoplasm of the oocyte during the early stages of fertilization; this can only be seen at high magnification using phase contrast microscopy.
Embryo Cleavage
The fertilized ova are transferred into a fresh equilibrated droplet of culture medium (occasionally supplemented with 15 to 20 percent of the patient’s decomplemented serum) and left in the incubator for another 24 to 36 hours. It is then examined for cell division before being transferred back into the uterus.
The embryo is judged to be developing normally in tissue culture if the dividing cells are approximately equal in size, uniformly regular in shape, homogeneous in appearance and occupying most of the space within the zona pellucida. Cell division must be progressive and occurs within a well defined interval of time. Thus, the embryo should contain two cells by 35 to 46 hours, four cells by 51 to 63 hours eight cells by 68 to 86 hours after insemination, and the sixteen cell stage should be attained by 84 to 112 hours after insemination (Edwards et al, 1980). If the cells of the developing embryoare irregular or shrink away from the zona pellucida or if the rate of cell division is markedly different from the rate described above, the embryo is judged to be abnormal and unsuitable for intrauterine transfer.
The following grading system (Puissant et al, 1987, Cohen et al, 1989) is used in our unit: Grade 1. Equal blastomeres with less than 10 percent fragmentation. Grade 2. Unequal blastomeres with less than 10 percent fragmentation. Grade 3. Equal or unequal blastomeres with 10 to 50 percent fragmentation. Grade 4. Equal or unequal blastomeres with more than 50 percent fragmentation.
Ovum Handling : The aspirated follicular fluid is directly examined under the magnifying power of a stereomicroscope (×10 to ×40) for the presence of oocytes. These are usually surrounded by one layer of nursing cells (the corona radiata) and both enclosed in a mass of granulosa cells (the cumulus oophorous). The oocyte-granulosa-cumulus complex (OGCC) measures about 1 mm in diameter and can readily be identified by the naked eye. The OGCC appears as a silvery shining dot suspended in the follicular fluid but must be confirmed by examination under the stereomicroscope to ensure that an ovum is embedded within the cumulus.
The OGCC is washed twice in the culture medium and is transferred into another droplet of equilibrated medium under sterile paraffin oil in a special Petri dish.The oocyte is also examined for signs of maturity and the time of insemination is determined accordingly. The insemination is usually performed 4 to 6 hours after oocyte retrieval, but this can be delayed for up to 24 hours if the oocyte is thought to be immature. Signs of maturity of the oocyte include the presence of the first polar body and the dispersion of the cumulus. An immature oocyte is tightly surrounded by the granulosa cells so that its internal details are not clearly recognized.
Sperm Preparation : After oocyte retrieval, the husband is asked to collect his ejaculate into a sterile container. The semen is allowed to liquefy at room temperature and a seminal fluid analysis is performed. In our program this is carried out using the computerized system of Fertipro (Autosperm) which calculates the sperm count, the motility grades (a, b, and c) as well as the velocity, linear velocity and linearity index (Chapter 9). A film of seminal fluid is also spread on a glass slide and stained arid strict morphology is calculated under oil immersion microscopy (x1000). If the normal forms calculated by strict morphology are higher than 14 percent, IVF is carried out. If these are less than 5 percent, ICSI is carried out (Chapter 19). If the normal forms are between 5 and 14 percent, IVF is carried out on half the oocytes and ICSI on the other half. The semen is then prepared by the swim-up method or the Percoll gradient method as described in Chapter 17 and the concentration recalculated. The sperm concentration is then adjusted to 0.5 to 1 million spermatozoa/mL).
Fertilization : A volume of 100 microlitres of the processed semen (containing 50,000 to 100,000 spermatozoa) is added to the droplet of medium containing the oocyte. Different culture media have been successfully used (e.g. Ham F10, Earle’s balanced salt solution, Menezo B2, etc.). They all consist of a balanced salt solution supplemented by essential amino acids and other nutrients. The osmolarity of the culture medium is about 280 mOsm/L and the pH is 7.4 if kept in an atmosphere containing 5% CO2. The culture is carried out in special polystyrene plastic tubes or dishes. If the culture is carried out in a dish, the medium is sometimes covered with a thin of sterile paraffin wax to minimize evaporation and hence keep the osmolarity constant.
The culture containing the oocytes and spermatozoa is transferred to a CO2 incubator which keeps the temperature at 37°C and an atmosphere of 5% CO2 in air. Alternatively an atmosphere of 5% CO2 + 5% O2 + 90% N2 is used. This ensures that the pH of the culture is kept at 7.4. Six to eighteen hours after insemination, the oocytes are denuded from the surrounding granulosa cells using finely pulled Pasteur pipettes and are then examined for signs of fertilization. At this stage, fertilization is confirmed by the finding of 2 pronuclei in the cytoplasm of the oocyte (the 2 PN stage, i.e. the 2 pronuclei stage). Other signs of fertilization are:
1. The appearance of an extruded second polar body in the vitelline space.
2. Retraction of the cytoplasm of the oocyte away from the zona pellucida.
3. The appearance of a sperm head, midpiece and tail inside the cytoplasm of the oocyte during the early stages of fertilization; this can only be seen at high magnification using phase contrast microscopy.
Embryo Cleavage
The fertilized ova are transferred into a fresh equilibrated droplet of culture medium (occasionally supplemented with 15 to 20 percent of the patient’s decomplemented serum) and left in the incubator for another 24 to 36 hours. It is then examined for cell division before being transferred back into the uterus.
The embryo is judged to be developing normally in tissue culture if the dividing cells are approximately equal in size, uniformly regular in shape, homogeneous in appearance and occupying most of the space within the zona pellucida. Cell division must be progressive and occurs within a well defined interval of time. Thus, the embryo should contain two cells by 35 to 46 hours, four cells by 51 to 63 hours eight cells by 68 to 86 hours after insemination, and the sixteen cell stage should be attained by 84 to 112 hours after insemination (Edwards et al, 1980). If the cells of the developing embryoare irregular or shrink away from the zona pellucida or if the rate of cell division is markedly different from the rate described above, the embryo is judged to be abnormal and unsuitable for intrauterine transfer.
The following grading system (Puissant et al, 1987, Cohen et al, 1989) is used in our unit: Grade 1. Equal blastomeres with less than 10 percent fragmentation. Grade 2. Unequal blastomeres with less than 10 percent fragmentation. Grade 3. Equal or unequal blastomeres with 10 to 50 percent fragmentation. Grade 4. Equal or unequal blastomeres with more than 50 percent fragmentation.

Embryo Transfer
Cleaved embryosare transferred into theuterine cavity. Most of the workers replace the embryos 48 hours after oocyte retrieval when they have reached the 2 to 4 cell stage, although many prefer to replace them after 72 hours when they have reached the 6 to 8 cell stage. The extended culture allows the selection of the better cleaving embryosthoughtto havea betterimplantation potential. Pregnanciescan alsooccur after the transfer of fertilized ova replaced as early as 24 hours when they are still at thepronuclearstage,although thesuccess ratesarelower (Hurst et al, 1998). This is termed “pronuclear stage uterine transfer”. In our program, transfer of 2 to 4 cell embryos at 48 hourspost-retrieval has been successfully carried out since its inception (Goswamy et al, 1984).
In preparation for transfer, the embryo is drawn gently in about 0.05 mL of culture medium, occasionally supplemented by 50 percent of the patient’s decomplemented serum, into a sterile catheter 1.4 mm in diameter. The catheter is then passed through the cervical canal and the embryos slowly released into the uterine fundus. To facilitate transfer, the catheter should be calibrated to gauge the position of its tip in the uterus. This step can also be confirmed by ultrasound guidance. The cervix should be handled minimally to avoid induction of uterine contractions and care should be taken not to injure the uterine fundus.
Different replacement catheters have been described but none has proven its absolute superiority. In our program, an Edward-Wallace catheter, a Frydman catheter or a Labotect catheter is used according to the findings of a trial replacement practiced during a previous cycle. After embryo replacement, the catheter should be examined under the microscope to confirm that the embryos have been properly placed in the uterus. Following embryo transfer, the patient is usually kept on the couch for 30 to 60 minutes, although recent studies have shown that this is not necessaryCleaved embryosare transferred into theuterine cavity. Most of the workers replace the embryos 48 hours after oocyte retrieval when they have reached the 2 to 4 cell stage, although many prefer to replace them after 72 hours when they have reached the 6 to 8 cell stage (Edwards et al, 1980). The extended culture allows the selection of the better cleaving embryosthoughtto havea betterimplantation potential. Pregnanciescan alsooccur after the transfer of fertilized ova replaced as early as 24 hours when they are still at thepronuclearstage,although thesuccess ratesarelower (Hurst et al, 1998). This is termed “pronuclear stage uterine transfer”. In our program, transfer of 2 to 4 cell embryos at 48 hourspost-retrieval has been successfully carried out since its inception (Goswamy et al, 1984).
In preparation for transfer, the embryo is drawn gently in about 0.05 mL of culture medium, occasionally supplemented by 50 percent of the patient’s decomplemented serum, into a sterile catheter 1.4 mm in diameter. The catheter is then passed through the cervical canal and the embryos slowly released into the uterine fundus. To facilitate transfer, the catheter should be calibrated to gauge the position of its tip in the uterus. This step can also be confirmed by ultrasound guidance. The cervix should be handled minimally to avoid induction of uterine contractions and care should be taken not to injure the uterine fundus.
Different replacement catheters have been described but none has proven its absolute superiority. In our program, an Edward-Wallace catheter, a Frydman catheter or a Labotect catheter is used according to the findings of a trial replacement practiced during a previous cycle. After embryo replacement, the catheter should be examined under the microscope to confirm that the embryos have been properly placed in the uterus. Following embryo transfer, the patient is usually kept on the couch for 30 to 60 minutes, although recent studies have shown that this is not necessary
Cleaved embryosare transferred into theuterine cavity. Most of the workers replace the embryos 48 hours after oocyte retrieval when they have reached the 2 to 4 cell stage, although many prefer to replace them after 72 hours when they have reached the 6 to 8 cell stage. The extended culture allows the selection of the better cleaving embryosthoughtto havea betterimplantation potential. Pregnanciescan alsooccur after the transfer of fertilized ova replaced as early as 24 hours when they are still at thepronuclearstage,although thesuccess ratesarelower (Hurst et al, 1998). This is termed “pronuclear stage uterine transfer”. In our program, transfer of 2 to 4 cell embryos at 48 hourspost-retrieval has been successfully carried out since its inception (Goswamy et al, 1984).
In preparation for transfer, the embryo is drawn gently in about 0.05 mL of culture medium, occasionally supplemented by 50 percent of the patient’s decomplemented serum, into a sterile catheter 1.4 mm in diameter. The catheter is then passed through the cervical canal and the embryos slowly released into the uterine fundus. To facilitate transfer, the catheter should be calibrated to gauge the position of its tip in the uterus. This step can also be confirmed by ultrasound guidance. The cervix should be handled minimally to avoid induction of uterine contractions and care should be taken not to injure the uterine fundus.
Different replacement catheters have been described but none has proven its absolute superiority. In our program, an Edward-Wallace catheter, a Frydman catheter or a Labotect catheter is used according to the findings of a trial replacement practiced during a previous cycle. After embryo replacement, the catheter should be examined under the microscope to confirm that the embryos have been properly placed in the uterus. Following embryo transfer, the patient is usually kept on the couch for 30 to 60 minutes, although recent studies have shown that this is not necessaryCleaved embryosare transferred into theuterine cavity. Most of the workers replace the embryos 48 hours after oocyte retrieval when they have reached the 2 to 4 cell stage, although many prefer to replace them after 72 hours when they have reached the 6 to 8 cell stage (Edwards et al, 1980). The extended culture allows the selection of the better cleaving embryosthoughtto havea betterimplantation potential. Pregnanciescan alsooccur after the transfer of fertilized ova replaced as early as 24 hours when they are still at thepronuclearstage,although thesuccess ratesarelower (Hurst et al, 1998). This is termed “pronuclear stage uterine transfer”. In our program, transfer of 2 to 4 cell embryos at 48 hourspost-retrieval has been successfully carried out since its inception (Goswamy et al, 1984).
In preparation for transfer, the embryo is drawn gently in about 0.05 mL of culture medium, occasionally supplemented by 50 percent of the patient’s decomplemented serum, into a sterile catheter 1.4 mm in diameter. The catheter is then passed through the cervical canal and the embryos slowly released into the uterine fundus. To facilitate transfer, the catheter should be calibrated to gauge the position of its tip in the uterus. This step can also be confirmed by ultrasound guidance. The cervix should be handled minimally to avoid induction of uterine contractions and care should be taken not to injure the uterine fundus.
Different replacement catheters have been described but none has proven its absolute superiority. In our program, an Edward-Wallace catheter, a Frydman catheter or a Labotect catheter is used according to the findings of a trial replacement practiced during a previous cycle. After embryo replacement, the catheter should be examined under the microscope to confirm that the embryos have been properly placed in the uterus. Following embryo transfer, the patient is usually kept on the couch for 30 to 60 minutes, although recent studies have shown that this is not necessary
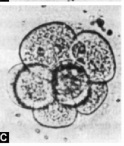
Embryo Freezing
The presence of 3 or more cleaving embryos poses an ethical as well as a practical problem. If all embryos are replaced, the risk of multiple gestations exists and this may end in pre-term delivery with its known complications including early neonatal death. This problem can be overcome by replacing a maximum of three embryos and the cryo-preservation of the rest.
Length of Culture before Embryo Freezing
There seems to be no difference in survival rates when the embryos are frozen at the 2, 4 or 8-cell stages. However, it has been suggested that pronuclear zygotes frozen at 26 hours after oocyte collection have a higher survival rate
The presence of 3 or more cleaving embryos poses an ethical as well as a practical problem. If all embryos are replaced, the risk of multiple gestations exists and this may end in pre-term delivery with its known complications including early neonatal death. This problem can be overcome by replacing a maximum of three embryos and the cryo-preservation of the rest.
Length of Culture before Embryo Freezing
There seems to be no difference in survival rates when the embryos are frozen at the 2, 4 or 8-cell stages. However, it has been suggested that pronuclear zygotes frozen at 26 hours after oocyte collection have a higher survival rate

IVF Checklist
1) Consents signed by both partners and witnessed by staff.
2) Financial agreement signed and payment scheduled.
3) Medication ordered and received.
4) Required labs drawn (both partners); this will include HIV, Hepatitis, and other sexually transmitted infections.
5) Patient instruction for medication and injection.
6) Patient nurse counseling visit to map projected IVF course.
7) Patient testing dates planned for ultrasound and labs.
8) Sperm sample frozen.
9) Arrange for someone to stay with you for the day of the egg retrieval and care for children if applicable.
10) Instructions regarding procedure arrival times and any other issues.
11) HCG trigger instruction and time assigned.
12) Begin antibiotic therapy as instructed. Now that you have a more complete picture of the path to IVF, it is hoped that you will feel more empowered and in control of your care. Feel free to ask any questions you may have, even if they seem trivial to you. It will reassure you to have answers and could be important to your cycle success. Good luck in your pursuit of having the family you’ve been longing for.
1) Consents signed by both partners and witnessed by staff.
2) Financial agreement signed and payment scheduled.
3) Medication ordered and received.
4) Required labs drawn (both partners); this will include HIV, Hepatitis, and other sexually transmitted infections.
5) Patient instruction for medication and injection.
6) Patient nurse counseling visit to map projected IVF course.
7) Patient testing dates planned for ultrasound and labs.
8) Sperm sample frozen.
9) Arrange for someone to stay with you for the day of the egg retrieval and care for children if applicable.
10) Instructions regarding procedure arrival times and any other issues.
11) HCG trigger instruction and time assigned.
12) Begin antibiotic therapy as instructed. Now that you have a more complete picture of the path to IVF, it is hoped that you will feel more empowered and in control of your care. Feel free to ask any questions you may have, even if they seem trivial to you. It will reassure you to have answers and could be important to your cycle success. Good luck in your pursuit of having the family you’ve been longing for.

Long Protocol
The long protocol mentioned above usually begins with baseline hormones and ultrasound done on approximately day three of your menstrual cycle. At this time your doctor will decide if birth control pills will be introduced. Typically, the first pill is begun on day three after labs and ultrasound have been completed. Your office should give you instructions on when to start and how to accurately take this medication. While on birth control pills, a second medication, called lupron or leuprolide in the generic equivalent, (GnRH agonist) will be introduced. It is the first injection that you as the patient will self- administer, usually in the morning. It is available in a premixed, multidose vial for ease of use. This medication further prepares the ovaries for egg stimulation and growth once fertility drugs have begun. It also acts to prevent ovulation while on fertility medication. You will be instructed when to start this drug and also when to stop the birth control pills. It is available in a premixed, multi-dose vial for ease of use. Once on leuprolide you will experience a menstrual period in approximately ten days. Once this menstrual period occurs you will be instructed to have an ultrasound and further hormone studies on a date assigned by your provider. Once ultrasound and lab results have been assessed by your physician and no problems found, your fertility drug treatment will begin. This process is called controlled ovarian hyperstimulation. This process encourages the production of multiple eggs, maximizing the likelihood of fertilization. Even though a woman usually produces one egg per month, controlled ovarian hyperstimulation as part of ART is routine. By producing multiple mature eggs for attempted fertilization in the laboratory, the odds increase for a successful pregnancy. At this point, you will begin frequent monitoring using ultrasound and blood draws to adjust dosage and provide safety.
Medications Used for Long Protocol By now, you are pro at self-injections. The injection process of these drugs is very similar. Your physician will decide which fertility drug will be used. While on the selected fertility medication you will also continue
The long protocol mentioned above usually begins with baseline hormones and ultrasound done on approximately day three of your menstrual cycle. At this time your doctor will decide if birth control pills will be introduced. Typically, the first pill is begun on day three after labs and ultrasound have been completed. Your office should give you instructions on when to start and how to accurately take this medication. While on birth control pills, a second medication, called lupron or leuprolide in the generic equivalent, (GnRH agonist) will be introduced. It is the first injection that you as the patient will self- administer, usually in the morning. It is available in a premixed, multidose vial for ease of use. This medication further prepares the ovaries for egg stimulation and growth once fertility drugs have begun. It also acts to prevent ovulation while on fertility medication. You will be instructed when to start this drug and also when to stop the birth control pills. It is available in a premixed, multi-dose vial for ease of use. Once on leuprolide you will experience a menstrual period in approximately ten days. Once this menstrual period occurs you will be instructed to have an ultrasound and further hormone studies on a date assigned by your provider. Once ultrasound and lab results have been assessed by your physician and no problems found, your fertility drug treatment will begin. This process is called controlled ovarian hyperstimulation. This process encourages the production of multiple eggs, maximizing the likelihood of fertilization. Even though a woman usually produces one egg per month, controlled ovarian hyperstimulation as part of ART is routine. By producing multiple mature eggs for attempted fertilization in the laboratory, the odds increase for a successful pregnancy. At this point, you will begin frequent monitoring using ultrasound and blood draws to adjust dosage and provide safety.
Medications Used for Long Protocol By now, you are pro at self-injections. The injection process of these drugs is very similar. Your physician will decide which fertility drug will be used. While on the selected fertility medication you will also continue